Development of π-Acidic Carbenes
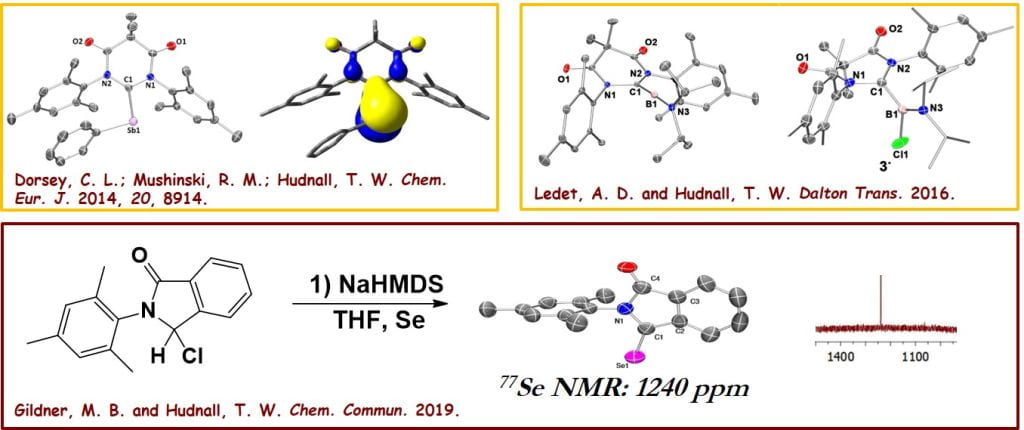
Our group has established a tradition in the design and synthesis of π-acidic carbenes that we have used for the stabilization of reactive main group element fragments and more recently in catalysis. In the realm of main group element chemistry, we have utilized primarily diamidocarbenes to prepare some of the first examples of carbene-supported stibinidenes and dicoordinated borylenes. As demonstrated in several publications, these π-acidic carbenes are also adept at stabilizing paramagnetic fragments owing to significant backbonding into their low-lying LUMOs. Our most recent efforts have focused on cyclic (aryl) amido carbenes which represent the most π-acidic carbenes reported to date.
Photochemistry of Diamidocarbenes
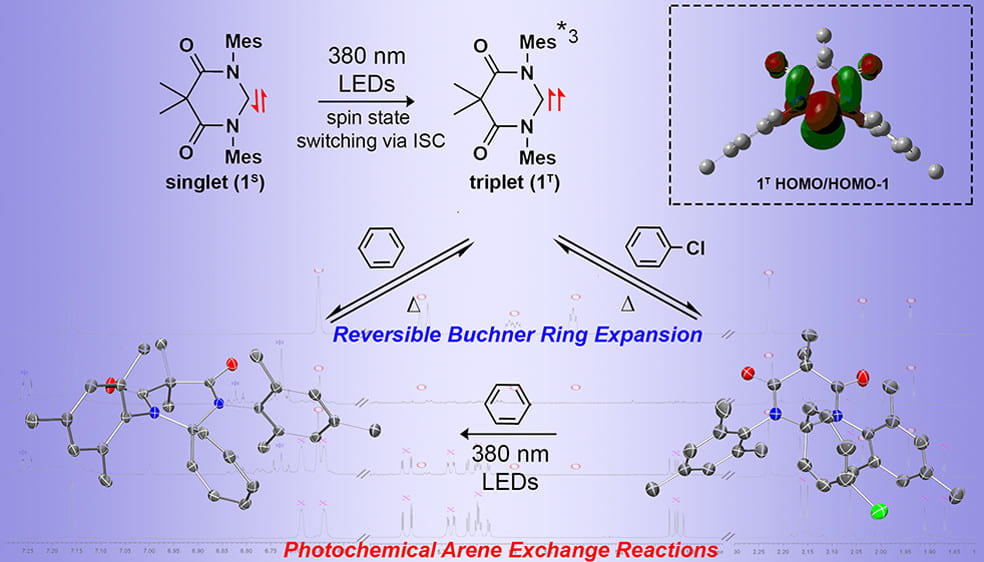
In 2017, our group stumbled upon unique photochemistry for diamidocarbenes. Our initial studies revealed that irradiation of the known 6-membered diamidocarbene with UV light results in excitation from a singlet ground to a triplet excited state. Using this triplet excited state, we have explored the reversible insertion into C=C bonds of aromatic substrates. These highly reversible Buchner ring expansion reactions were the first ever reported. More recently have have begun exploring additional photochemical reactions of the diamidocarbene with extended polyaromatic hydrocarbons as well as unactivated alkanes.
Main Group Element Catalysis
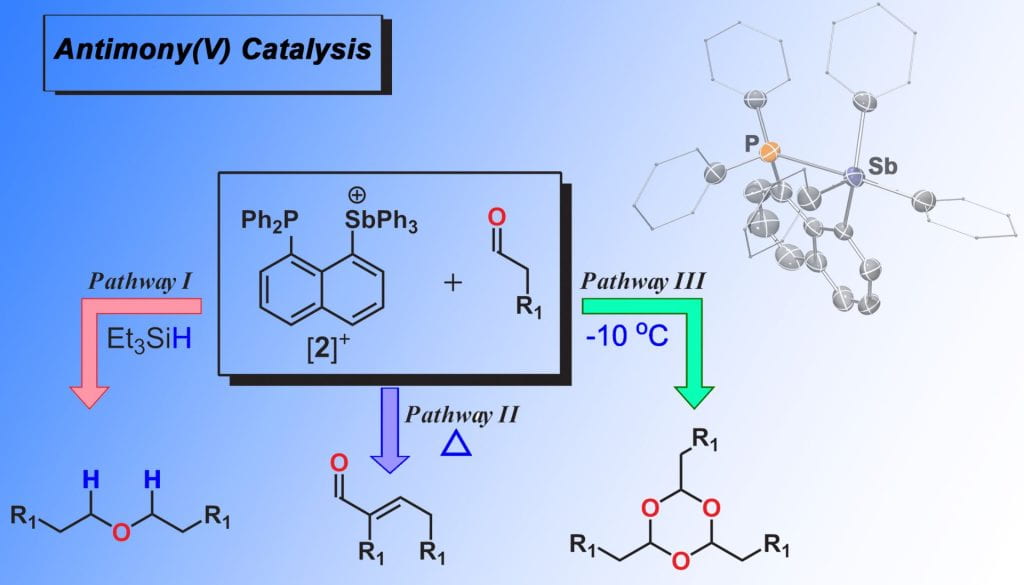
For some time our group has been interested in exploiting main group elements as catalysts. Our early efforts focused on using heavier main group elements such as Sb(V) stibonium centers as simple Lewis acid catalysts. We demonstrated that such compounds are competent for i) the catalytic reductive coupling of a variety of aldehydes into symmetric ethers using Et3SiH as a reductant, ii) to selectively catalyze the Aldol condensation reaction to afford α-β unsaturated aldehydes when aldehydes with 2 α-hydrogen atoms were used, adn iii) catalyzed the cyclotrimerization of aliphatic and aromatic aldehydes to afford the industrially-useful 1,3,5 trioxanes. More recently we have turned our attention to utilizing our π-acidic carbenes as ligands to chalcogenide centers to enhance catalytic activity. In these systems, the main group element center is metallomimetic in that these catalysts operate under typical 2-electron transformations, i.e. oxidative addition/reductive elimination.